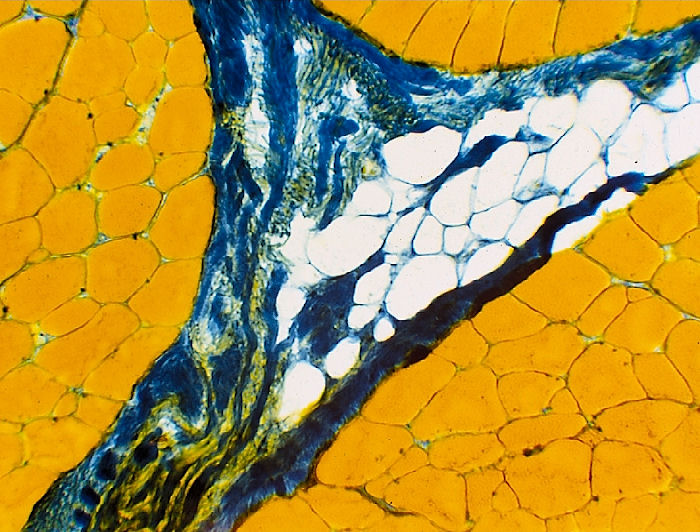

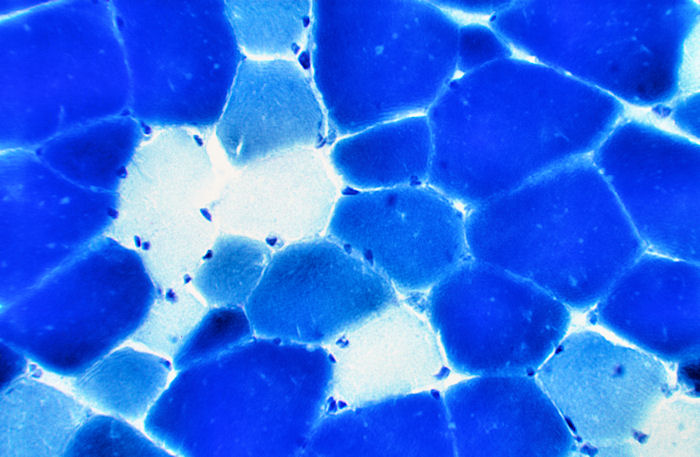
Myofibrillar ATPase Stain
This stain allows a clear discrimination between
three fiber types, one slow-twitch fibre, type I (white fibres) and
two fast-twitch fiber types, types IIA (light blue) and IIB (dark blue).
Procedure:
1. Cut tissue sample on a microtome 10 to 12
mm
thick and place onto microscope slides (microscope slides should be
pre-rinsed/cleaned in an ethanol solution).
Leave slides at room temperature for at least 5 min.
2. Wash twice for 1 min each with Tris-Ca2+ (Pre-Rinse
Solution).
3. Incubate for 5 min in the Alkaline Pre-Incubation Solution.
4. Wash twice for 30 sec each with Tris-Ca2+ (Pre-Rinse
Solution).
5. Incubate for 90 min at 37 °C in the Incubation Solution.
6. Wash 4 times for 20 – 30 sec each in CaCl2 Wash Solution.
7. Rinse for 3 min in a 2% CoCl2 Solution (mix immediately before
use).
8. Rinse 4 times for 20 – 30 sec each in dH2O.
9. Stain for 28 sec in a 1% Azure Stain (mix immediately before use, see
notes).
10. Rinse continuously under tap water for 5 min to rinse off remaining
Azure Stain.
11. Rinse once with dH2O.
12. Wash with:
50 % Ethanol for 5 to 10 sec
70% Ethanol for 5 to 10 sec
96% Ethanol for 5 to 10 sec
absolute Ethanol for 1 to 2 min
13. Fix sections using a 1:1 (v:v) solution of Xylol:absolute Ethanol (dispose of in proper receptacle after use).
14. Mount a cover slip using a non-aqueous cover slip medium (i.e.
Histofluid).
Solutions and Reagents:
Pre-Rinse
Solution
(Fresh Daily) 100 mL 0.18 M CaCl2
(pH 7.3)
12.1 g Trishydroxymethylaminomethane
add dH2O to 1000 mL
Alkaline Pre-Incubation Solution
10 g CaCl2
(pH 10.4 with 1 N NaOH)
7.44 g Glycine
100 mL Formaldehyd (37%)
add dH2O to 1000 mL
Incubation Solution (Fresh Daily)
20 mL 0.1 M Glycine Buffer
(pH 9.4, warm to 37 °C)
10 mL 0.18 M CaCl2 (2%)
0.152 g ATP
add dH2O to 100 mL
0.1 M Glycine Buffer
125 mL Glycine (7.51g/250 mL dH2O)
(pH 9.4)
42 mL 0.4 M NaOH (8 g/250 mL dH2O)
add dH2O to 500 mL
0.18 M CaCl2
19.98 g CaCl2 in 1000 mL dH2O
CaCl2
Wash Solution
13.3 g CaCl2 in 1000 mL dH2O
Notes:
- Azure A is the most commonly used for this purpose. The 1% Azure A stain is made by dissolving 1 g of Azure A in 100 mL water.
- Unless otherwise noted solutions are to be kept at 4 °C, are good for up to one month, and can be disposed of down the sink.
Reference
Brooke, M. H., and K. K.
Kaiser. 1970. Muscle fiber types: how many and what kind ? Arch.
Neurol. 23:369-379.
Szentkuti, L., and A. Eggers.
1985. Eine
zuverlässige Modifikation der Myosin-ATPase-Reaktion zur histochemischen
Darstellung von drei Fasertypen in der Skelettmuskulatur von Schweinen.
Fleischwirtsch. 65:1398-1404.